 |
 |
| Physiology? | Figures & Illustrations | Test Questions | Daily Quiz | Calculators | Physiology Tutor | Glossary |
 |
 |
 |
 |
 |
 |

Secondary Active Transport
Secondary active transport is a form of active transport across a biological membrane in which a transporter protein couples the movement of an ion (typically Na+ or H+) down its electrochemical gradient to the uphill movement of another molecule or ion against a concentration/electrochemical gradient. Thus, energy stored in the electrochemical gradient of an ion is used to drive the transport of another solute against a concentration or electrochemical gradient. The ion moving down its electrochemical gradient is referred to as the driving ion because it is movement of this ion that drives the uphill movement of another ion/molecule (driven ion/molecule). Secondary active transport is also commonly referred to as ion-coupled transport and, in fact, coupling between the driving and driven species is obligatory. That is to say that both the driving and driven species must be bound to the transporter for translocation across the membrane to occur. Unlike in primary active transport in which ATP hydrolysis provides the free energy needed to move solutes against a concentration gradient, in secondary active transport, the free energy needed to perform active transport is provided by the concentration gradient of the driving ion. To call this process secondary active transport is appropriate since the existence and maintenance of the concentration gradient of the driving ion is accomplished by primary active transporters (i.e., pumps). Sodium serves as the driving ion in many (but not all) secondary active transporters located in the plasma membrane of various cells. This is appropriate as there is a steep Na+ concentration gradient across the plasma membrane that is maintained by the Na+/K+/ATPase. In mammals, the extracellular Na+ concentration ([Na+]o) is around 145 mM and the intracellular Na+ concentration ([Na+]i) is around 15 mM. The free energy stored in this gradient is used by Na+-coupled transporters to drive the uphill movement of substrates.
Two types of secondary active transport: Cotransport and Exchange
Two variations of secondary active transport exist: cotransport (also known as symport) and exchange (also known as antiport). The transport proteins responsible for secondary active transport are referred to as secondary active transporters and are specifically referred to as cotransporters (also known as symporters) and exchangers (also known as antiporters). Cotransport and cotransporters are also commonly written as co-transport, and co-transporters, respectively. Sodium is the driving ion for many cotransporters and exchanger and, therefore, these transport proteins may also be referred to as sodium-coupled cotransporters. Similarly, there are many examples of proton-coupled cotransporters and exchangers. Figures 1 and 2 provide a summary of these secondary active transport processes.

Figure 1. Secondary active transport.
In secondary active transport, the movement of a driving ion down an electrochemical gradient is used to drive the uphill transport of another ion/molecule against a concentration or electrochemical gradient. Two types of secondary active transport processes exist: cotransport (also known as symport) and exchange (also known as antiport). In cotransport, the direction of transport is the same for both the driving ion and driven molecule/ion, whereas in exchange, the driving ion and driven ion/molecule are transported in opposite directions. X and Y represent transporter substrates. Na+, sodium; K+, potassium; ATP, adenosine triphosphate; ADP, adenosine diphosphate; Pi, inorganic phosphate.
In cotransport, the direction of transport is the same for both the driving ion and driven ion/molecule. For example, the Na+/glucose cotransporter (SGLT1), found in the small intestine and kidney proximal tubules, simultaneously transports 2 Na+ ions and 1 glucose molecule into the cell across the plasma membrane. In contrast, in exchange, the driving ion and driven ion/molecule move in opposite directions. For example, the Na+/Ca2+ exchanger (NCX), found in cardiac muscle cells and elsewhere in the body, transports 3 Na+ ions into the cell in exchange for 1 Ca2+ ion transported out of the cell. Not all cotransporters utilize Na+ as the driving ion. Some use an existing proton electrochemical gradient. For example, the H+/oligopeptide transporter (PepT), found in the small intestine, couples the downhill movement of H+ across the plasma membrane to the uphill transport of dipeptides and tripeptides into the cell against a concentration gradient.
Not all secondary active transporters are found in the plasma membrane. For example, H+/neurotransmitter exchangers, found in the membrane of synaptic vesicles in axon terminals, utilize the proton electrochemical gradient across the vesicle membrane to drive the uphill transport of neurotransmitter into the vesicle (Fig. 2). In all cases, the electrochemical gradient of the driving ion is maintained by primary active transporters. The Na+ gradient across the plasma membrane is maintained by the Na+/K+/ATPase and the proton gradient across the synaptic vesicle membrane is maintained by the H+/ATPase.

Figure 2. Secondary active transport across vesicular membranes.
Secondary active transporters may also be localized to the membrane of internal organelles. For example, H+/neurotransmitter exchangers use the H+ electrochemical gradient (proton electromotive force) established by the V-type H+/ATPase to drive neurotransmitter molecules against a concentration gradient into the lumen of synaptic vesicles. NT, neurotransmitter; H+, proton; ATP, adenosine triphosphate; ADP, adenosine diphosphate; Pi, inorganic phosphate.
Transport coupling stoichiometry determines the effectiveness of substrate transport against a concentration gradient
For both cotransporters and exchangers, the effectiveness of the transport process can be defined by the concentrative capacity of the transport process. Concentrative capacity is a measure of how well the driven ion/molecule is concentrated against a concentration gradient. For example, for the Na+/glucose cotransporter, concentrative capacity can be expressed as the ratio of intracellular to extracellular glucose concentration ([Glucose]i/[Glucose]o). This ratio indicates how well the Na+/glucose cotransporter can concentrate glucose inside of the cell against a concentration gradient. Concentrative capacity is strictly related to the ion/substrate coupling stoichiometry of the transporter per transport cycle. For example, the coupling stoichiometry of the intestinal Na+/glucose cotransporter is 2 Na+ ions to 1 glucose molecule per transport cycle. That is to say that there is thermodynamic coupling between Na+ and glucose cotransport. For any given transporter, the coupling stoichiometry is usually a fixed ratio. A higher ion/substrate coupling ratio (e.g., 3:1 vs. 2:1) leads to a significantly higher concentrative capacity of transport.
Cotransporters and exchangers may be electrogenic or electroneutral
A consequence of the ion/substrate coupling ratio is that the activity of some cotransporters and exchangers leads to the translocation of net charge across the membrane. A transporter that leads to the net translocation of charge across the membrane is said to be electrogenic. For example, with a coupling stoichiometry of 2 Na+ ions to 1 glucose/galactose molecule, Na+/glucose cotransport by SGLT1 represents an electrogenic process. Such charge movements across the membrane lead to small electrical currents and, therefore, electrophysiological methods may be used to measure the activity of electrogenic transporters. When no net charge is transported across the membrane per transport cycle, the process is said to be electroneutral. Figures 3 and 4 provide a few examples of cotransporter proteins, and Fig. 5 provides examples of exchanger proteins.
Direction of transport is determined by the electrochemical or concentration gradient of the driving ion/species
While the physiological concentration gradient of the driving ion normally determines the direction of transport in cotransporters and exchangers, under experimental conditions, cotransporters and exchangers may work in reverse if the concentration gradient of the driving ion is reversed. For example, if the intracellular and extracellular concentrations of Na+ are experimentally reversed, the Na+/glucose transporter would utilize this gradient to transport 2 Na+ ions and 1 glucose molecule out of the cell (provided that the glucose substrate is present inside of the cell). Generally, the coupling stoichiometry of transport is the same whether the transporter works in the forward or reverse mode. Moreover, if the experimental conditions are designed to create a sufficiently high concentration of the transported substrate, the transporter can then utilize this artificial concentration gradient to drive the uphill transport of the normally designated driving ion! For example, leaving the normal inwardly-directed Na+ electrochemical gradient intact, if the cytoplasmic glucose concentration is experimentally made to be very high, then glucose would in fact act as the driving species to drive SGLT-mediated cotranslocation of 2 Na+ ions and 1 glucose molecule out of the cell! Therefore, secondary active transporters can be thought of as thermodynamic machines that respond to the concentration gradients of the driving ion as well as the driven ion/molecule. Physiologically, the driven ion/molecule is moved against a concentration gradient, but experimentally, its concentration gradient can be defined to make it the driving ion/molecule! This point is described later when examining the thermodynamics of the Na+/glucose cotransporter.
Examples of cotransporters
A few examples of cotransporter proteins are shown in Figures 3 and 4. Please note that this is only a partial list of cotransporters.

Figure 3. Examples of cotransport proteins.
Cotransport proteins (cotransporters or symporters) are found in many different cells and tissues and perform a variety of important physiological functions. Six examples are shown here. See text for details. Na+, sodium; K+, potassium; Cl-, chloride; I-, iodide; Pi, inorganic phosphate.
The Na+/glucose cotransporter (SGLT1) is found in the apical membrane of epithelial cells of the small intestine and renal proximal tubules. It utilizes the Na+ electrochemical gradient to drive the uphill transport of glucose into the cell. As mentioned above, the ion/substrate coupling stoichiometry of SGLT is 2 Na+ ions 1 glucose molecule (Fig. 3). Therefore, SGLT1 is an electrogenic transporter. In addition to glucose, SGLT1 recognizes galactose as a substrate. Its function is crucial to dietary glucose and galactose absorption in the small intestine and reabsorption of filtered glucose and galactose in the proximal tubule of kidney nephrons.
The Na+/phosphate cotransporter (NaPi) is found in the apical membrane of epithelial cells of the small intestine and renal proximal tubules. It utilizes the Na+ electrochemical gradient to drive the uphill transport of inorganic phosphate (Pi) into the cell. For every transport cycle, NaPi (IIa and IIb) couples the inward translocation of 3 Na+ ions and 1 divalent Pi (HPO42-) (Fig. 3). Thus, NaPi types IIa and IIb are electrogenic transporters. A third type, NaPi-IIc (not shown here), is electroneutral with a coupling stoichiometry of 2 Na+ to 1 divalent Pi (HPO42-).
The Na+/I- symporter (NIS) is responsible for the accumulation of iodide by the thyroid gland. It is localized to the basolateral membrane of thyroid follicular cells and co-translocates 2 Na+ ions and 1 I- ion per transport cycle (Fig. 3). NIS is also found in other tissues such as in the mammary glands, where it functions to transport iodide into the nursing mother's milk.
The Na+/K+/2Cl- cotransporter (NKCC), the Na+/Cl- cotransporter (NCC), as well as the K+/Cl- cotransporter (KCC) belong to the same family of transporters (Fig. 3). The Na+/K+/2Cl- cotransporter (NKCC) utilizes the Na+ electrochemical gradient to drive the inward cotranslocation of Na+, K+, and Cl- with a 1 Na+ : 1 K+ : 2 Cl- stoichiometry. Among other places, NKCC is found in the apical membrane of the thick ascending limb of the loop of Henle, where it performs an important role in urine concentration. The so-called "loop diuretics" block the activity of NKCC. The Na+/Cl- cotransporter (NCC) utilizes the Na+ electrochemical gradient to drive Cl- transport into the cell with a 1 Na+ : 1 Cl- coupling stoichiometry. NCC is found in the apical membrane of the epithelial cells of kidney nephron distal tubules and, in fact, is the target of thizaide diuretics. The K+/Cl- cotransporter (KCC) cotranslocates 1 K+ and 1 Cl- out of the cell per transport cycle. Here, the driving ion is K+. Remember that the activity of the Na+/K+/ATPase creates a large outwardly directed K+ electrochemical gradient ([K+]i = 150 mM and [K+]o = 4 mM). As apparent from the coupling stoichiometry ratios shown in Fig. 3, NKCC, NCC, and KCC are all electroneutral cotransporters.

Figure 4. Additional examples of cotransport proteins.
Six additional examples of cotransporters are shown. See text for details. R-COO- represents monocarboxylates such as lactate, pyruvate, acetoacetate, and β-hydroxybutyrate. Na+, sodium; H+, proton; Cl-, chloride; HCO3-, bicarbonate; GABA, γ-aminobutyric acid.
The transporter for the inhibitory neurotransmitter γ-aminobutyric acid (GABA) belongs to a large family of Na+- and Cl--coupled transporters. The GABA transporter (GAT) regulates the basal concentration of GABA in the nervous system and, in addition, regulates the concentration and lifetime of GABA in the synaptic cleft. The GABA transporters couple the inward translocation of both Na+ and Cl- to the simultaneous inward translocation of GABA with a coupling stoichiometry of 2 Na+ : 1 Cl- : 1 GABA (Fig. 4). Thus, the GABA transporters are electrogenic. Other transporters that belong to this family are transporters for the neurotransmitters serotonin, dopamine, and norepinephrine, as well as transporters for the osmolytes betaine and taurine.
The H+/monocarboxylate cotransporter (MCT) recognizes monocarboxylates such as lactate, pyruvate, acetoacetate, and β-hydroxybutyrate. It is a proton-driven transporter with a coupling stoichiometry of 1 H+ : 1 monocarboxylate and, thus, MCT is an electroneutral transporter (Fig. 4). The direction of transport is governed by the prevailing proton gradient.
The H+/oligopeptide transporter (PepT) is located in the apical membrane of the small intestine epithelial cells and renal proximal tubules. It couples the downhill movement of 1 H+ to the uphill movement of 1 dipeptide or 1 tripeptide (Fig. 4). In addition, PepT serves as the entry route for peptidomimetic drugs such as β-lactam antibiotics. Stomach emptying of acidic chyme into the early regions of the small intestine creates an acidic environment with a favorable transmembrane proton gradient for PepT-mediated transport of di/tripeptides. PepT is thought to be the dominant player in nitrogen absorption in the small intestine and nitrogen reabsorption in the kidney tubules.
Sodium-coupled bicarbonate cotransporters (NBC) perform important acid-base balance functions. Both electroneutral (NBCn) and electrogenic (NBCe) isoforms have been identified (Fig. 4). Remarkably, the NBCs belong to the same family as Cl-/bicarbonate exchagers discussed below (AE). For the electrogenic isoforms, the coupling stoichiometry appears to vary depending on the tissue in which the transporter is expressed. The direction of transport may be inward or outward. For example, NBCe1 located in the basolateral membrane of kidney proximal tubules, mediates the outward translocation of 1 Na+ ion and 3 HCO3- ions into the interstitial fluid, where the reabsorbed bicarbonate is returned to the circulation by entering the peritubular capillaries. Thus, NBCe1 plays a crucial role in renal bicarbonate reabsorption.
Examples of exchangers
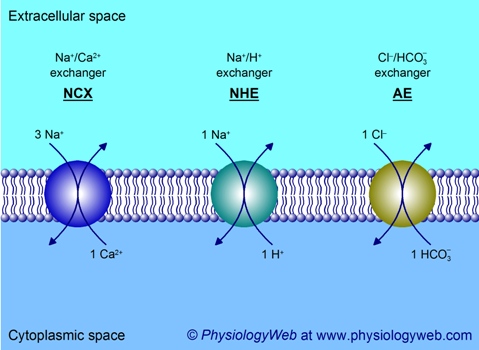
Figure 5. Examples of exchangers.
Exchangers (also known as antiporters) are found in many different cells and tissues and perform a variety of important physiological functions. Three examples are shown here. See text for details. Na+, sodium; Ca2+, calcium; H+, proton; Cl-, chloride; HCO3-, bicarbonate.
The Na+/Ca2+ exchanger (NCX) is ubiquitously found in many cells and tissues and plays an important role in cytoplasmic Ca2+ homeostasis. It couples the inward movement of 3 Na+ ions down the Na+ electrochemical gradient to the uphill movement of 1 Ca2+ ion against a very steep electrochemical gradient (Fig. 5). Remember that resting Ca2+ concentration values in the cytoplasm and extracellular fluid are 70 nM and 2 mM, respectively. NCX is electrogenic.
A ubiquitously found exchanger that plays a key role in the regulation of cytoplasmic pH is the Na+/H+ exchanger (NHE). Here again, the Na+ electrochemical gradient is utilized by the exchanger to extrude H+ from the cell with a 1 Na+ : 1 H+ coupling stoichiometry (Fig. 5). Thus, NHE is electroneutral.
Another exchanger that is widely distributed is the Cl-/bicarbonate exchanger (also referred to as anion exchanger or AE). The coupling stoichiometry of transport is 1 Cl- to 1 HCO3- and, therefore, the exchanger is electroneutral (Fig. 5). As mentioned above, these exchangers belong to the same family as Na+-coupled bicarbonate cotransporters (NBC). The direction of transport by the Cl-/bicarbonate exchanger is governed by the concentration gradients of Cl- and bicarbonate. For example, in red blood cells (erythrocytes) travelling in systemic capillaries, excess production of bicarbonate in the cytoplasm (as a result of CO2 hydration reaction catalyzed by carbonic anhydrase) drives the outward translocation of HCO3- in exchange for inward translocation of Cl-. When the red cells pass through the lung capillaries, the direction of transport is reversed such that inward translocation of HCO3- is coupled to outward translocation of Cl-. The HCO3- that enters the cells ultimately gives rise to CO2 (catalyzed by carbonic anhydrase), which then leaves the cell and ultimately the capillaries to enter the air-filled alveoli of the lungs, where it is then exhaled into the atmosphere.
Cotransporters and exchangers represent many gene families in the human genome
The examples of cotransporters and exchangers given above do not take into consideration that many of the transporters discussed have multiple isoforms and that features such as tissue localization, coupling stoichiometry, substrate specificity, etc. may vary among the isoforms. Moreover, there may be differences among ortholog transporters (i.e., species differences such as human versus rat or mouse). For a complete list of secondary active transporters found in the human genome, please visit the Human Genome Organization (http://www.hugo-international.org/) nomenclature page (http://www.genenames.org/).
Posted: Thursday, February 10, 2011
Last updated: Wednesday, June 29, 2011
Last updated: Wednesday, June 29, 2011